E. COLI
Typical Applications
- Recombinant Protein Therapeutics
- Plasmids
Advantages
- Simple, Fast And Easy
- Very High Protein Expression


Gainesville, FL (March 8, 2023) Evanoa Bioscience Inc., a cell line developer for biologics manufacturing, together with Aldevron®, a global leader in the custom development and manufacture of plasmid DNA, RNA and proteins for the biotech industry, today announced the signing of a licensing agreement for two E. coli strains to be used in plasmid production.
 BIOSCIENCE
BIOSCIENCE
EVANOA uses a novel proprietary technology to make order-of-magnitude improvements in cell lines used for the
production of
biopharmaceuticals such as recombinant proteins, viral vaccines, gene therapies and
plasmids.
Our technology and expertise applies to any cell line or industrial condition and allows our partners to increase yield, reduce extraction costs and enhance the overall process efficiency, giving them an insurmountable advantage over their competitors.
The FDA has approved seventeen cell lines for the production of vaccines and biotherapeutics. EVANOA has already improved key performance indicators on five cell lines and started developments on the other twelve. EVANOA will keep monitoring FDA approvals for its future cell line developments.
Typical Applications
Advantages
Typical Applications
Advantages
Typical Applications
Advantages
Typical Applications
Advantages
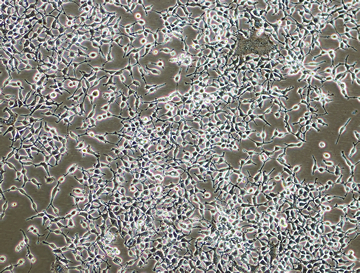
Typical Applications
Advantages
We can start a collaboration at multiple stages of your development. If our partners are still creating their cell banks we can explore our catalog of improved microorganisms that have already been successfully used in tests or scaled production. If our partners have already finalized their cell banks, we could improve their cell factories for specific traits.

Cell factories with
higher yield or specific traits



We are located in Alachua, FL but have partners all over the globe.